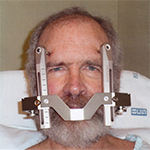
 Me trying to smile with a metal frame screwed into my skull
Me trying to smile with a metal frame screwed into my skull
This post will mainly interest those who have to cope with advanced cancer or a similar problem.
Following the discovery of a brain metastasis, while I was in LA last month trying to get into a clinical trial, I saw I had three hills to climb. The first hill was surgical removal of the tumor. That was handled expeditiously at home in North Vancouver. The second hill was radiation therapy to reduce the chances of recurrence within the brain. The third would be to get into another clinical trial for an anti-PD-1 immunotherapy drug. I have now climbed the second hill.
Of the three, this one seemed to have the most uncertainty. The standard of care here in BC is whole-brain radiation therapy (WBRT). But I didn’t like what I read about WBRT. Mistrusting alarmist internet sites, I concentrated on peer-reviewed clinical studies, such as “Neurocognitive function impairment after whole brain radiotherapy for brain metastases: actual assessment,” published in 2012 in Radiation Oncology. This survey paper references many studies of cognitive decline following WBRT. Its key findings are (1) worrisome statistics showing cognitive impairment have been gleaned from records of surviving patients during the months and years following WBRT, and (2) these studies fail to distinguish deterioration due to WBRT from deterioration due to other causes. Other causes include the brain cancer itself (a problem shared by everyone who getsWBRT), cancer in other parts of the body, chemotherapy and other medications, other radiotherapy, and prior neurological disease (strokes and such). So, we know that brain deterioration follows WBRT, but we do not know how much of it is caused by WBRT.
That is shocking, when you consider that WBRT is the standard of care. Since Hippocrates, the first rule of medical ethics is, “Do no harm.” That has been interpreted to allow doctors to do some harm if necessary to avoid greater harm. But not knowing how much harm a treatment causes makes the rule hard to apply.
Claudia and I started investigating alternatives. One, which attracted me at first, was to do nothing—just observe and hope for the best. This would avoid all bad effects of radiation, while re-qualifying me sooner for an anti-PD-1 trial. (The trials I want most call for a four-week washout period following radiation.) But I could find few credible sources supporting this idea. I had fourteen days to kill before my appointment (the earliest I could get) with my local radiation oncologist, so I decided to seek a second opinion. A phone call to Seattle, credit card in hand, got me a consultation with a radiation expert, with two days notice. Claudia and I drove down for the day, with a long list of questions. The main one was what type of radiation treatment would be best for me: no treatment (observation), whole-brain, or stereotactic radio-surgery, which would treat the surgical cavity and spare the rest of my brain. Secondary questions concerned the damage caused by WBRT and how it could be mitigated: lower fractional doses, radio-sensitizing drugs designed to boost the radiation’s damage to tumors, radio-protective drugs intended to make healthy cells less vulnerable to radiation, hyperbaric oxygen therapy either just before treatment (to reduce hypoxia of melanoma cells and make them more radio-sensitive) or afterwards (to promote healing), and shielding to protect my hippocampus, damage to which could impair my ability to lay down memories. We had an hour of face time with the expert (comparing favourably with the 15 minutes max. we ever get to talk to a doctor in Canada) and found him well informed, able to shed light on most of our questions.
It was good to get the perspective of his clinical experience. Radio-sensitizers? They’ve been talked about for decades at oncology conferences, but nothing much has come of them. Memantine to protect neurons against radiation? It would be nice if it worked. One thing he offered was to spread out the radiation in smaller doses: instead of the standard regimen of 30 Gy in 10 daily fractions, he would give me 40 Gy in 20 fractions (2 Gy daily). Petri-dish studies show neurons recovering better from 2 Gy than the 3 Gy daily dose. But the definitive studies to prove this have not been done. (And are unlikely to be done. Clinical studies are almost all funded by companies who stand to profit when their new products are approved by government. Trials comparing different ways of applying an old treatment, like radiation therapy, don’t often happen.) He added that cost squeezing by US insurance companies meant that his clinic would almost certainly have to conform to the 30 Gy/10 fractions practice in a year or so, even though, in his opinion, it was a lower standard of care.
Some aspects of my case argued for the stereotactic approach: the fact that there only appeared to be a single brain metastasis, and the gamble that I might be one of those who would survive long enough to be declared in a state of remission. My chances were improved by my general health (excellent, if you don’t count the metastases elsewhere in my body) and the existence of the anti-PD-1 drug trials. Anti-PD-1’s are a game changer for melanoma, so far showing an objective response in about 50% of patients.
Near the end of the interview, we asked, “What would you do, if it were you?” “Whole brain,” was the answer, followed by a thoughtful pause as the doctor looked me over. “But in your case, the argument for stereotactic becomes more compelling.”
Still on the fence.
We also talked to a melanoma patient who had brain metastases treated by surgery and WBRT in 2010. Her tumors were much bigger than mine—the size of lemons. (She was also much younger, and pregnant at the time.) Now in mid-2013, both mother and toddler are doing well. I heard no signs of brain damage in our conversation. But how could I know? Another thing that is not done, outside of trials, is formal testing of cognitive function before and after brain radiation.
I ran it by my regular oncologist, who said I was asking a lot of high-level questions, and the radiation oncologists would have to “duke it out”.
I emailed my other oncologist, the one running the trials in LA. He had a definite opinion. “I would go with SRS [stereotactic radiosurgery] here as it saves you the risk of long term morbidity. Also the fact that the lesion was so big and there were no others makes me think that this is a solitary lesion without other smaller ones below limit of detection lurking.” This was what I wanted to hear. So when my appointment with the local radiation oncologist rolled around, I was mostly decided. But not quite. I was prepared to hear new information that might change my mind.
Metastatic melanoma, she reminded me, is a very serious disease—especially in the brain. I had already read, more than once, that doctors haven’t worried much about thelong-term effects of WBRT because most patients don’t survive long enough for it to matter.
But WBRT was all she offered. She advised me strongly against observation only—advice I hardly needed at this point. The dose would be the standard 30 Gy in 10 fractions. Shielding the hippocampus was not available. Protective drugs would not be considered. What she could provide was strictly limited by the approved protocols, which left little room for discretion.
I told her about my researches and my consultation with the Seattle doctor. I asked, “Is there any good reason I might not know about why I shouldn’t go with stereotactic?” She surprised me a little by not arguing, other than to point out that if I had it done in Seattle, I’d have to pay for it. Then she asked how much. “Pricey, but manageable. $22,000.” In the end, she gave it her blessing. We parted on good terms and the understanding that if I ever needed whole-brain RT, I could come back to her for it.
That meeting took place July 8. On July 9, Claudia and I were in Seattle, ready for an MRI early on the 10th. This MRI would be higher-resolution than the one I’d had in LA, taking images of 1 mm slices of my brain rather than the usual 5 mm. Its purpose was two-fold: to plan the path of the stereotactic radiosurgery, and to look for additional brain metastases. That afternoon, we were to meet the radiosurgeon for consultation.
At lunch (very nice Vietnamese at Ba Bar; I had a zesty salad and catfish on a vermicelli bed, as fresh-tasting as my server promised; it’s a good idea to consult your server before ordering) Claudia grew apprehensive about the afternoon meeting. Lately, all the results of scans and tests had been worse than expected. I reminded her that a couple of lumps I worried about in 2012 turned out to be harmless seromas. She said she was developing a phobia about it. I said it’s really hard to predict the future.
I still think that.
Before meeting the doctor, we were thoroughly briefed by a nurse who told me what to expect during the Gamma Knife procedure, scheduled for 6:30 in the morning. I could have a light breakfast. I would have a metal frame screwed into four points of my skull. (See picture.) The pain would be managed by local injections of lidocaine; but the lidocaine itself would sting at first. I’d be given dexamethasone against inflammation, and Ativan to keep me calm . After the frame was attached, I’d have a quick CT scan for the purpose of registering the previous day’s MRI to the frame, and thence to my skull. This precise spatial registration would allow the Gamma Knife to work with sub-millimetre accuracy, zapping only the thin margin of the surgical cavity and nothing beyond. The whole procedure would last about four hours. I could listen to music if I wanted. I would probably go to sleep. When the nurse mentioned the MRI I couldn’t help asking if it showed any surprises. From overhearing the doctors’ discussion, she thought not. I could feel Claudia start to relax.
Then we met the radiosurgeon who was working out the plan of attack—referring to the MRI to program the Gamma Knife. She showed us the images. I was in luck; there were no new hot spots. The area to be treated was easy to see. She explained how the Gamma Knife works, as well as a related technology called the Cyber Knife, using computer simulations. We went on to review my previous MRI, and a PET scan done last year. She spent three-quarters of an hour with us, answered all our questions, including the critical one– “How many of these do you do?” “About two a day.”—and offered various followups as part of the deal. I felt it was $22,000 well spent.
Claudia and I had the rest of the afternoon off. We went for a long walk in a park, then went out for an excellent dinner at Toulouse Petit, a restaurant our son Tom picked out for us. It was like a date night.
The day of the Gamma Knife went as advertised.. The frame was screwed in, which hurt, but not for long. Then it felt like a hat—a metal hat that was way too tight. Touching the frame produced the odd sensation that it was part of my body, as though I’d grown antlers or an exoskeleton. If anything touched the frame, the noise sounded very loud.
I was discharged at 10:30 AM, paper report (operation successful!) and disk in hand. The MRI came to a whopping 43 GB. Claudia drove home, and later drove me to my meeting with a committee of my photo club planning a major competition we will host next year. I was delighted to be able to participate.
I’ve reached the second summit, and am now looking at the third—getting into another clinical trial. I’ve emailed my oncologist in LA, who encouraged me to come back. I’m waiting for his answer. But Claudia has already been on the phone exploring trial possibilities elsewhere.
In a situation like mine, having a loving spouse take on the role of case manager is a huge boon. If a study were done (it won’t be), I’m sure it would show a contribution to overall survival (OS).
Those are all the facts, the ones that matter. Now that I have to deal with the future again, I must rely on my imagination.
The second hill wasn’t as arduous as I expected, and I still feel reasonably fresh. Surveying the third, I don’t have a clear sense of distance; and there are clouds around the peaks. Nor do I know the depth of the valley separating the ridge I’m on from the next upward slope.
When making plans for my future self, I like to think of him in the third person. It makes more than a grammatical difference; it helps me maintain an evenhanded attitude (commonly called perspective). I think about him, a guy a lot like me—quite likeable (although sometimes maddeningly thick), someone who can be trusted to look out for the people I love, a guy whose politics I approve of—whose days may turn out much better or worse depending on what decisions I make now. Indeed, his life may depend on my decisions, so I take them seriously. I feel affection towards him, and want things to go as well for him as possible. As for Claudia, and for my children and other people I know.
So I peer across the valley, trying to plan his route. The bits of slope I can make out look steep. There is too much cloud. I don’t feel confident he’ll make it to the top—find a trial he qualifies for. Perhaps he could find another way forward—a second course of ipilimumab—but whether it would be approved is uncertain, and whether it would work unlikely, given that the first course failed to prevent new tumors. I must assume he can climb the mountain. I know it has at least two peaks. If he is eligible for a clinical trial, it is likely to have more than one arm. Patients in one of those arms will get old-style chemotherapy. That peak is exposed, bleak and dangerous. He would probably be offered paclitaxel and carboplatin (P&C). The statistics on P&C for melanoma are discouraging; seven months into one study of 61 patients, 60 of them were dead. The other peak holds more promise—a mountain hut, with a stove and firewood and canned goods, where he could take shelter and recover strength—that is, anti-PD-1 immunotherapy, with an objective response rate of 50%. It’s obvious which peak he should aim for; but I can’t tell him which direction to go. If he is accepted into a trial, whether he gets into the chemo arm or the anti-PD-1 arm depends on the randomization run done by the drug company. If he wound up in the wrong arm, he could withdraw—but if he did that, he would disqualify himself for future trials of that drug. If he accepted the chemo, he would be on that craggy, hostile peak with no clear way to get off. He might get off—be given the anti-PD-1 drug—if his cancer ‘progressed’ (got worse), and he was declared to have failed the chemo. That would come with a loss of time, loss of strength, a heavier burden of disease.
And of course, even if he makes his way to the right peak and finds the hut, he can’t stay there forever. The territory that lies beyond remains unknown. A 50% chance of a gentle descent into a friendly valley. A 50% chance of barren terrain and ever-worsening conditions.
How to advise him? The mountain hut is the best bet. As to the safest way to get there, I don’t know. I will try to find out more. He will have to pick out much of the path as he goes along. The trail is poorly marked. I wish him well.
Return to the Phantom Self home page.
